INTRODUCTION
Water is the commonest compounds
everyone might have come across,
covering about 70% of the earth. If you
have seen, tasted, felt or smelled water
before, you may easily describe water as
being colourless, tasteless, or odourless.
Technically, water is a compound of two
atoms of hydrogen and an atom of oxygen;
expressed as H2O.
Sources of water
Water can be obtained from:
1. The sea
2. Lakes
3. Rivers
4. Lagoons
5. Rain
6. Wells
7. Pipe-borne/ taps
Physical properties
1. Water is colourless, tasteless,
odourless.
2. It boils at 100oC and freezes at 0oC.
3. It is neutral, hence has a pH of 7.
4. It has a maximum density of 1gcm-1
at
4
oC.
5. It expands when heated from -4
oC and
0
oC and contracts when melted from
0
oC to 4oC. This is the reason why ice
floats on water; ice has lower relative
density.
6. Water has a high specific heat.
7. It conducts heat more easily than any
liquid except mercury. This fact causes
large bodies of water, like lakes and
oceans, to have essentially a uniform
vertical temperature profile.
8. Its molecules exist in liquid form over
an important range of temperature from
0
oC - 100°C. This range allows water
molecules to exist as a liquid in most
places on our planet.
9. It is a universal solvent. It is able to
dissolve a large number of different
chemical compounds. This feature also
enables water to carry solvent nutrients
in runoff, infiltration, groundwater flow
and living organisms.
10. Water has a high surface tension. In
other words, water is adhesive and
elastic, and tends to aggregate in drops
rather than spread out over a surface as
a thin film. This phenomenon also
causes water to stick to the sides of
vertical structures despite gravity's
downward pull. Water's high surface
tension allows for the formation of
water droplets and waves, allows plants
to move water (and dissolved nutrients)
from their roots to their leaves, and the
movement of blood through tiny
vessels in the bodies of some animals.
Determining the boiling point of water
Pour water into a clamped boiling
tube.
Put a thermometer in the water.
Record the reading on the
thermometer.
Heat the setup for two minutes and
note how the reading on the
thermometer rises as the water heats
up.
Note the temperature reading when
the water begins to boil.
(Determining the boiling point of water)
Observation
It would be observed that when the water
starts to boil the temperature reading will
be 100 oC and remain so no matter how
long the water boils. This shows that the
boiling point of water of water is 100 oC.
Chemical properties of water
1. Water reacts with metallic oxides to
form alkaline solutions and hydrogen
gas.
For example:
CaO + 2H2O → 2CaOH + H2
2. Water reacts with non-metallic oxides
to form acidic solutions.
For example:
H2O + SO2 → H2SO3
3. Water reacts with metals to form metal
oxides and give off hydrogen gas.
For
example:
2H2O + 2K → 2KHO + H2
4. Anhydrous white copper (II)
tetraoxsulphate (VI) reacts with water
to form blue pentahydrate salt.
CuSO4+ 5H2O → CuSO4.5H2O
5. Dry blue cobalt (II) chloride reacts with
water to form red or pink hexahydrate
salt.
CoCl2 + 6H2O → CoCl2.6H2O
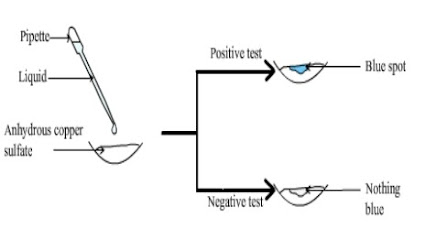
Test for water
Not all liquids are or contain water; and a
solid substance may contain water which is
not observable with the naked eye. A Test
for water must be used to identify water.
Testing whether a liquid is water
Add a drop of the liquid to white
anhydrous copper sulphate.
The spot where the water droplet fell
turns blue immediately.
This shows that the liquid is or contain
water.
(Testing whether a liquid is water)
Testing for water in a solid
Put a sample of anhydrous copper
sulphate on the solid.
The area turns blue.
This shows that the solid contains
water.
(Testing for water in a solid)
HARDNESS AND SOFTNESS OF
WATER
How does water become hard or soft? You
might ask. Well, since water is such a good
solvent, during its journey from its source
it dissolves and picks up some compound
such as calcium, magnesium or iron
compounds and other impurities. These
compound and impurities affect the
properties of water and may make it be
hard.
Hard water
Water is said to be hard if it does not
lather easily with soap.
Such water usually contains magnesium,
Mg+
, calcium, Ca+
or iron (III), Fe+ ions.
Hard water does not lather because the ions
react with the soap to form an insoluble
scum.
Soft water
Soft water is the water which lathers
readily with soap.
Since soft water does not contain dissolved
ions which interfere with the action of
soap, it lathers easily with soap.
Types of hard water
There are two types of hard water, namely:
Temporal hard water
Permanent hard water
Temporal hard water
This is the type of hardness caused by the
presence of dissolved calcium hydrogen
carbonate, (Ca(HCO3)2 in the water.
This occurs when rainwater, which
contains dissolved atmospheric carbon (II)
oxide, comes in contact with limestone,
calcium carbonate, CaCO3, as it runs
through rocks or soil which contains the
compound (CaCO3).
This reaction can be
expressed as:
CaCO3 + H2O + CO2 → Ca(HCO3)2
Removal of temporary hardness
1. By boiling or heating
When temporary hard water is heated, the
reaction that causes it is reversed. That is
calcium hydrogen carbonate, Ca(HCO3),
reverses to form a white solid insoluble
calcium trioxocarbonate (IV), CaCO3,
carbon dioxide, CO2 and water, H2O.
The
reaction is given as:
Ca(HCO3) → CaCO3 + CO2 + H2O
2. By adding calcium hydroxide
A calculated amount of lime, calcium
hydroxide, can be added to temporary hard
water to cause calcium carbonate to float
on the water, which can be filtered off
easily. The equation is given as;
Ca(OH)2 + Ca(HCO3)2 → 2CaCO3 + 2H2O
Permanent hard water
Permanent hardness is caused by dissolved
calcium, Ca+
, magnesium mg+
or iron Fe+
ions. The above ions are contained in salts
such as CaSO4, MgSO4 or FeSO4, which
are contained in rocks. When runoff water
comes into contact with these salts, the
ions dissolve and flow into water sources.
Permanent hardness cannot be removed by
boiling, but by chemical means.
(Scale formed by hard water on heating
element of kettle)
Removing or softening permanent
hardness in water
1. Distillation
When water is boiled, it vaporizes. The
vapour can then be condensed or cooled
back into pure water in another container,
leaving the ions that cause hardness in the
original container.
2. Addition of washing soda, Na2CO3
Sodium carbonate removes soluble calcium
and magnesium ions as insoluble calcium
or magnesium carbonate (CaCO3 or
MgCO3) that float on the surface of the
water and can easily be filtered off. The
reaction is given as:
Na2CO3 + Ca2+
→ CaCO3 + 2Na+
3. Addition of caustic soda, NaOH
NaOH removes soluble calcium and
magnesium ions as insoluble hydroxides.
The equation is given as:
2NaOH + Ca2+
→ Ca(OH)2 + 2Na+
4. De-ionization or ion exchange
This is the use of a special device known as
ion exchanger to remove the ions, e.g.
Ca2+, Mg+
, responsible for hardness by
exchanging them for other ions that do not
cause hardness such as sodium, Na+ ion.
(Ion exchange process)
Testing for hardness in water
Fill a beaker with sample of water from
a river.
Add some drops of liquid soap to the
water and stir or shake it.
Check whether the water lathers.
Boil the solution and add and a few
drops of liquid soap to it.
Compare the lathering ability of the
water before and after boiling.
Check the beaker for scum (white
powdery substance).
Observation
If the water lathers more after heating
then it means its hardness is temporary. If
the water forms scum in the beaker and
does not lather, then its hardness is
permanent.
A control experiment can be setup with a
sample of distilled water.
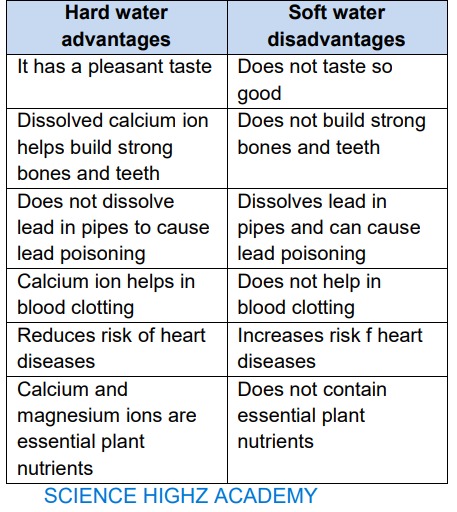
ADVANTAGES AND
DISADVANTAGES OF HARD AND
SOFT WATER
(Advantages of soft water over hard
water)
(Advantages of hard water over soft
water)
TREATMENT OF WATER FOR
PUBLIC CONSUMPTION
Pure water is not found in nature.
Chemicals, bacteria, and suspended
sediment particles enter the water through
exposure to air and runoff. Before
arriving at your tap, raw water is treated to
eliminate the presence of harmful bacteria
and unpleasant coloration, taste, and odour.
The Stages in large-scale water
treatment
1. Preliminary Treatment
Preliminary treatment or pre-treatment is
any physical, chemical or mechanical process used on water before it undergoes
the main treatment process.
During
preliminary treatment:
screens may be used to remove rocks,
sticks, leaves and other debris;
chemicals may be added to control the
growth of algae;
pre-sedimentation stage can settle out
sand, grit and gravel from raw water.
2. Coagulation
After preliminary treatment, the next step
is coagulation. Coagulation removes
small particles that are made up of
microbes, silt and other suspended
materials in the water. Treatment
chemicals such as alum are added to the
water and mixed rapidly in a large basin.
The chemicals cause small particles to
clump together (coagulate). Gentle mixing
brings smaller clumps of particles together
to form larger groups called floc. Some of
the floc begins to settle during this stage.
3. Flocculation
During the flocculation stage, the heavy,
dense floc settles to the bottom of the water
in large tanks. As you can imagine, this can
be a slow process. Once the floc settles, the
water is ready for the next stage of
treatment.
4. Clarification/ sedimentation
Clarification occurs in a large basin where
water is again allowed to flow very slowly.
Sludge, a residue of solids and water,
accumulates at the basin's bottom and is
pumped or scraped out for eventual
disposal. Clarification is sometimes called
sedimentation.
5. Softening and Stabilization
When water is too hard (i.e. contains too
much calcium, magnesium or other
minerals), it forms scale and causes a
variety of problems in pipes. Hard water
can also result in laundering and washing
problems, because it reduces the
effectiveness of soaps and detergents.
Conversely, when too many of these
minerals are removed, water can become
too soft. Soft water can cause corrosion in
pipes.
Drinking water plants attempt to maintain a
desirable balance between hardness and
softness. This is accomplished by adding
minerals to soft water and removing them
from hard water.
6. Filtration
Turbidity is a physical characteristic that
makes water appear cloudy when
suspended matter is present. The filtration
process removes suspended matter, which
can consist of floc, microorganisms
(including protozoan cysts such as Giardia
and Cyrptosporidium), algae, silt, iron, and
manganese precipitates from ground-water
sources, as well as precipitants which
remain after the softening process.
These suspended materials are filtered out
when water passes through beds of
granular material, usually composed of
layers of sand, gravel, coal, garnet, or
related substances.
7. Chlorination & Disinfection
Chlorine is fed into the water system as
either a dry powder or in solution. During
disinfection, disease-causing organisms are
destroyed or disabled. Chlorine is normally
used because it is economical and rapid. It
is important to add the right amount of
chlorine at the water treatment plant to
make sure disinfection continues while the
water is flowing through the distribution
system.
8. Storage
Finished water (the term water treatment
professionals use) is stored in holding
tanks. The tanks provide a water reserve to meet the changing water demands of the
communities they serve.
(Treatment of water for public consumption)
( Summary of public water treatment
procedure)
Small-scale water treatment
In our homes, school etc, we can still treat
impure water for our own consumption.
The following are some methods of
purifying water on a small scale:
1. Boiling
Raw water can be boiled to kill bacteria
and other microbe in it. Boiling is a very
effective water treatment method, but can
only be done on a small scale because of
the cost involved if it is done on a large
scale to serve a town or community.
2. Filtration
Suspended particles on the surface of the
water can be filtered off with a water filter
or fine mesh. Filtration can be done before
or after boiling, and can be done a couple
of time to ensure that the water is free of
all suspended particles.
3. Sedimentation
Water which contains sediments or
suspending particles can also be allowed to
stand for some time in order to allow all
the particles to settle at the bottom.
4. Distillation
Another efficient way of purifying water is
distillation. Here, water is boiled, and the
vapour is condensed into another container
leaving the impurities in the original
container.
(Distillation process)
Importance or uses of water
Domestic use – At home, school, etc.
water is used for drinking, washing,
bathing, flushing toilet etc.
Energy production –- Hydroelectric
plants capture the kinetic energy of falling
water to make electricity. This is done with
a dam. The dam forces the water level to
go up so that the water will have more
power when falling. The force of the
falling water pressing against turbines'
blades causes them to spin. The spinning
turbines transmit the kinetic energy of the
falling water to generators. The generators
spin when the turbines spin generating
electricity that will be transmitted on the
power lines to homes, schools and
businesses.
(A hydroelectric power dam)
Plants and animals – Plants and animals
cannot exist without water. Plants need
water to prepare their food which animals
depend on.
Agricultural use -- Water is used for
irrigation, spreading fertilizers, herbicides,
and pesticides, etc.
Industrial use – Water is used for
cooling heavy duty machines and washing
equipments. It is an important element in
many products like chemicals, drugs,
lotions, shampoos, cosmetics, cleaners and
beverages. Water is used in processing
food and in innumerable factories and
industrial processes including the
manufacturing of paper.
TEST QUESTIONS
1. a) What is the chemical formula for water?
b) Mention five sources of water.
c) List three physical and three chemical properties of water.
2. a) Write a short note on the following:
i) hard water
ii) soft water
b) Explain three methods for softening permanent hard water.
c) How can boiling remove temporary hardness in water?
3. a) Give three advantages and three disadvantages each of hard and soft water.
b) What are the causes of permanent and temporary hardness in water.
c) Differentiate between hard water and soft water.











No comments:
Post a Comment